how to draw an atom model
Questions and Answers
How do I make a model of an atom?
Do you lot need to make a model or a drawing of an atom for science course? If so, follow these instructions to learn where all of the atom'south pieces become.
Step 1 - Gather Data
Before you can build your model, yous volition demand to know how many protons, neutrons and electrons your atom has. If yous do non already know how to employ the Periodic Table of Elements to discover this information, read the 'How many protons, electrons and neutrons are in an cantlet of...?' page to learn how.
Permit's use nitrogen as an example. Using data found on the Periodic Table of Elements, we tin tell that an boilerplate cantlet of nitrogen contains 7 protons, vii neutrons and vii electrons.
Step ii - Gather Materials
Now that you know how many protons, neutrons and electrons you will need for your model, information technology is fourth dimension to decide what to use to represent them. Ping-pong assurance, rubber balls, ball bearings, golf balls and styrofoam balls have all been used in the past. Basically, anything that is roundish and that tin can exist glued to each other should work. It is helpful if the balls are color coded so that it is easier to tell which balls are protons, which are neutrons and which are electrons. It is too helpful if the electrons are smaller than the protons and neutrons.
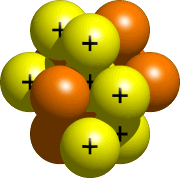
Step three - Build the Nucleus
The nucleus, the key part of the atom, is fabricated from protons and neutrons. All of your atom's protons and neutrons get in the nucleus. For nitrogen, the nucleus would wait something like this:
Step 4 - Place the Electrons
The electrons are found exterior the nucleus. How you place them depends on which model of atomic construction your grade is studying. In that location are a few ways this can exist done:
The Planetary Model
This model depicts an earlier view of the structure of the atom, presently subsequently the nucleus was discovered. This model is typically taught to younger students as an introduction to atomic structure. In it, the electrons are said to orbit the nucleus much similar the planets in the solar system orbit the sun. Depending on your instructor, the actual orbits commonly don't matter. Having the right number of electrons is usually practiced plenty. A planetary model of a nitrogen atom could look something similar this:
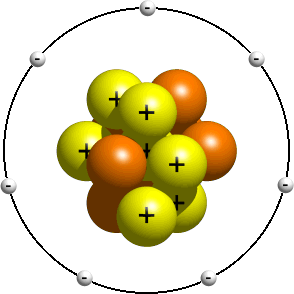
In the planetary model, a nitrogen atom has a central nucleus, composed of vii protons and seven neutrons, surrounded by seven electrons.
The Bohr Model
Scientists before long realized that the planetary model was an inaccurate description of atomic structure. They learned that electrons could simply occupy sure orbits (ordinarily referred to every bit energy levels or shells) around the nucleus. They also discovered that only a certain number of electrons could fit in each energy level.
To properly place the electrons around the nucleus, you will demand to refer to your chemical element's electron configuration tabular array. To discover your chemical element's electron configuration table, go to the Periodic Tabular array of Elements, click on your element and scroll to the bottom of the page. If you practise not know how to read the electron configuration tabular array, read the 'How practise I read an electron configuration table?' page for assistance.
Co-ordinate to nitrogen'southward electron configuration table, an cantlet of nitrogen contains 2 electrons in its first energy level and v electrons in its second energy level. A Bohr model of a nitrogen atom could look like this:
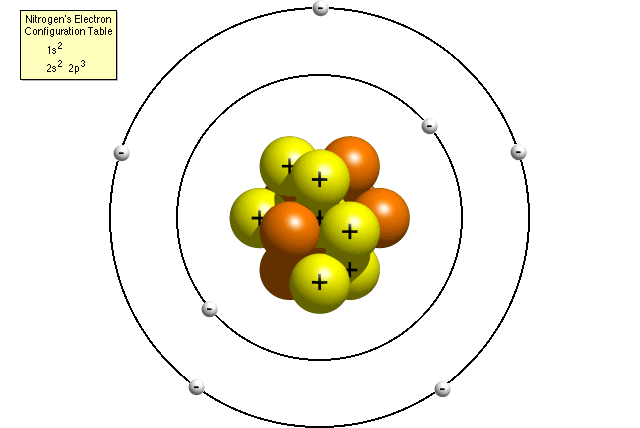
In the Bohr model, a nitrogen atom has a fundamental nucleus, composed of vii protons and vii neutrons, surrounded by seven electrons. Two of the electrons are in the first free energy level while the other 5 are in the second energy level.
The Refined Bohr Model
Further studies showed that the Bohr model wasn't as accurate every bit it could exist. Scientists learned that each energy level is made from a sure number of sub-shells. The sub-shells, which are named s, p, d and f, can each concur only a certain number of electrons. The s sub-shell can only hold 2 electrons, the p sub-beat tin hold vi, the d sub-crush can hold 10 and the f sub-shell can concord 14. The number of available sub-shells increases as the energy level increases.
To properly place the electrons around the nucleus, you will need to refer to your chemical element'southward electron configuration table. To find your element's electron configuration table, go to the Periodic Table of Elements, click on your element and roll to the bottom of the folio. If you practice not know how to read the electron configuration table, read the 'How do I read an electron configuration table?' page for help.
According to nitrogen'south electron configuration table, an atom of nitrogen contains two electrons in its first energy level (both in the due south sub-shell) and 5 electrons in its second energy level (two in the s sub-beat out and 3 in the p sub-shell). A more than accurate Bohr model of a nitrogen atom could look like this:
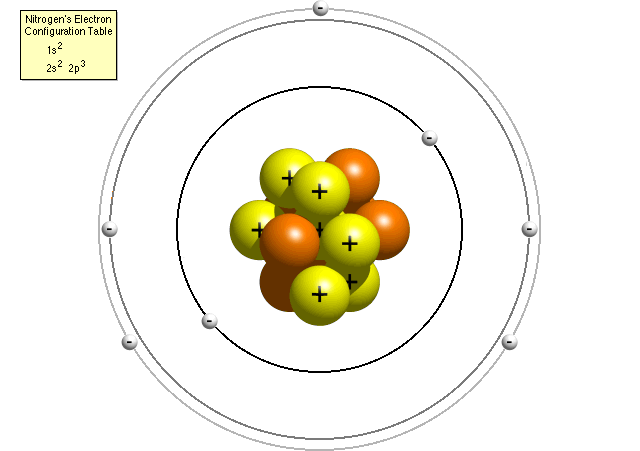
In the refined Bohr model, a nitrogen atom has a central nucleus, equanimous of seven protons and 7 neutrons, surrounded by seven electrons. Two of the electrons are in the s sub-beat of the outset energy level, two are in the south sub-shell of the second free energy level and three are in the p sub-shell of the second energy level.
Step v - Things to Call back
It is important to think that a model is a simplified representation of an object. Some of the models discussed above are more authentic than others, only none of them are completely right. Here are a couple of the things we accept ignored:
The Size of the Nucleus
In the drawings above, the nucleus is too large. Or, put some other way, if the nucleus is going to be that large, the electrons are too close. Real atoms are mostly empty space. If nosotros wanted our drawings to be accurate, we would accept to place the electrons virtually a mile abroad. Clearly, it would be difficult to bring a cartoon that big to form.
Electrons exercise not Orbit the Nucleus
In the drawings higher up, we have fatigued dainty circles showing where the electrons become effectually the cantlet. In reality, scientists cannot tell exactly where an electron is at a given moment or where it is going. They can calculate the probability that an electron will exist constitute in a given volume of space, but that isn't the same equally knowing where that electron is. This beliefs is described in the Breakthrough Model of the atom. Although it is the most authentic description that scientists currently have of the cantlet, it is much more hard to sympathise.
Related Pages:
Source: https://education.jlab.org/qa/atom_model.html
Posted by: bunchhollices.blogspot.com


0 Response to "how to draw an atom model"
Post a Comment